
Application Note
CloneSelect Imager FL fluorescence for rapid day zero monoclonality
- Day zero monoclonality assurance
- Early rejection or acceptance of clones
- Confirm and track various CRISPR edits with multichannel fluorescence detection – label-free reporting
- High-speed acquisition to meet the demands of high-throughput applications
- Preconfigured algorithms for assessment and reporting monoclonality
- Compatible with a variety of plate formats from 6-well to 384-well microplates
Prathyushakrishna Macha, Ph.D. | Research Scientist | Molecular Devices
Introduction
Gene editing allows DNA manipulation, i.e., addition, deletion, or modification using different tools. Several nucleases for genome editing are now available: zinc finger nucleases (ZFN), transcription activator-like effector nucleases (TALEN), meganucleases, and the CRISPR (clustered regularly interspaced short palindromic repeats) associated protein 9 (Cas9). Targeted gene editing, especially with CRISPR, has revolutionized science and has many applications in various fields, mainly in cell line development, cell and gene therapy.
The human p53 gene can eliminate cellular damage caused due to stress induced by internal or external factors. The growth inhibitory properties of p53 keep the damaged DNA in cells from replicating. The role of p53 also extends to cell cycle arrest, senescence, and apoptosis activities in a cell. The need to better understand p53’s function in humans has motivated many studies probing its role in toxicology, pharmacology, and cancer studies, to name a few.
Gene editing to obtain a cell line, like the p53 knockout described here, involves several steps1 . The steps involve stable transfection, edited cell pool generation, single cell printing, and screening for a stable protein-expressing monoclonal cell line. Effective optimization of steps using these right tools contributes to an efficient process of cell line development. This in turn leads to a cut down in the time, effort, and costs of various scientific advances to help accelerate R&D, revolutionize drug discovery, cure disease, expand gene-edited crop production, and so on.
The CloneSelect® Imager FL (CSI-FL) offers great assistance in upstream process development of cell line development workflows. The stringent regulations of cell line development monoclonality can be easily met using the fluorescence detection capabilities of CSI-FL to confirm a single cell and to provide a day zero assurance of monoclonality. The cells seeded into the wells of a microplate for monoclonality screening, using single-cell printing (SCP) or limiting dilution4 usually need to be single in the count and express the protein of interest.
Here we present a fluorescent method for identifying monoclonal HEK-293 cells after gene editing with CRISPRbased p53 knockout plasmids that have a red fluorescent protein (RFP) marker for puromycin selection. The edited cells were seeded into 96-well plates with a single-cell printer. These were then monitored using CSI-FL to assess monoclonality and monitor growth through RFP and white light channels.
The fluorescent method of detection rules out the detection of debris, dust, air bubbles and unwanted cells. It screens high-value clones during the imaging process. Fluorescence to screen viable healthy cells could be achieved through our cell-specific fluorescent dye (Cell Tracker Green CMDFA) or a label-free approach i.e., expression of fluorescence protein as a marker via gene inserts.
The Fusion software of the CSI-FL analyzes the cell counts and the presence of a fluorescence marker for the edited gene. Each well is analyzed through a robust single-cell identification technique using fluorescent images2,3.
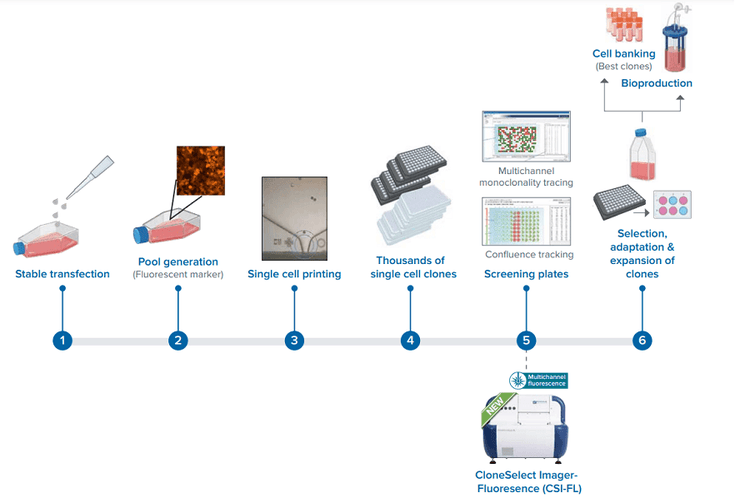
Cell line development workflow using our new CSI-FL.
Materials and Materials
HEK-293 cells from ATCC were maintained as per instructions before they underwent TP53 knockout using the Santa Cruz p53 CRISPR/Cas9 knockout (KO) plasmid and p53 homology-directed repair (HDR) plasmid following the manufacturer’s instructions (Santa Cruz Biotechnology) with the TransIT-X2 delivery system (Mirus Bio) and 24h post-transfection cells were selected with puromycin. The puromycin concentration was 1.5 μg/mL for the first 72 hours and then gradually tapered to 0.5 μg/mL through a media change for regular maintenance until stable line generation1 .
This pool at 1 million cells/ mL in serum-free media was used to single-cell print 96/ 384 well plates using CloneSelect® Single-Cell Printer, f.Sight. These plates were then monitored on day 0, followed by days 1, 4, 6, 7, and 11 to record growth using white light and an RFP channel of CSI-FL (4X objective). These cells were further imaged using The ImageXpress® Micro Confocal High-Content Imaging System (IXM-C) to get more detailed images of the cells (10X objective). Once grown to confluence they were transferred into a 12-well plate → 6-well plate → 25-cm2 flask → 75-cm2 flask and then frozen for further experiments.
Results
The HEK-293 cells were successfully transfected with the KO plasmid and with HDR for puromycin selection expressed RFP (long term) and this pool of polyclonal cells was further screened for monoclonal cell line development. This was done by single-cell printing of the cell pool (Figure 1) into 96 well / 384 plates, later selected with Puromycin (tapering concentration, for better growth), and scaled up for further assays. The use of fluorescence in CSI-FL and plate wells layout made it easy to locate the cells and monitor the single cells in individual wells of a 96-well plate for expansion (Figures 2 & 3). The cells imaged on CSI-FL (4X magnification) were imaged on IXM-C for higher (10, 20, 40X) magnifications (Figure 5). CSI-FL provided a rapid high-throughput reading of multiple plates using multiple channels within a couple of hours.
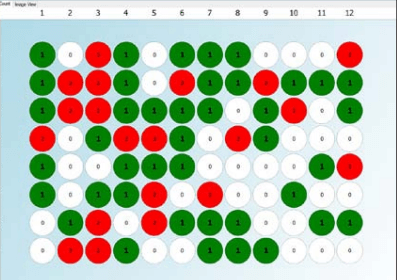
Figure 1. Day zero monoclonality report for a single cell printed 96-well plate generated using CSI-FL fluorescence imaging and Fusion software (Green – one cell detected in the well, Grey – no cell detected, Red – more than one cell detected)
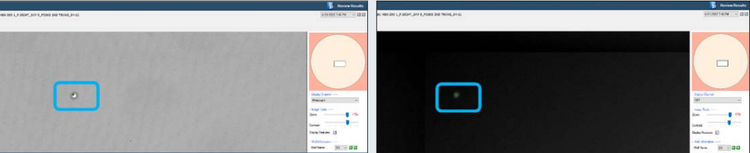
Figure 2. CSI-FL Fusion software white light (left) and RFP (right) channel image view of well D3 from plate 1 on Day 0 in Figure 1. Monoclonality assurance plate report. The green dot (bracketed) on the cell is the Fusion software cell count representation.
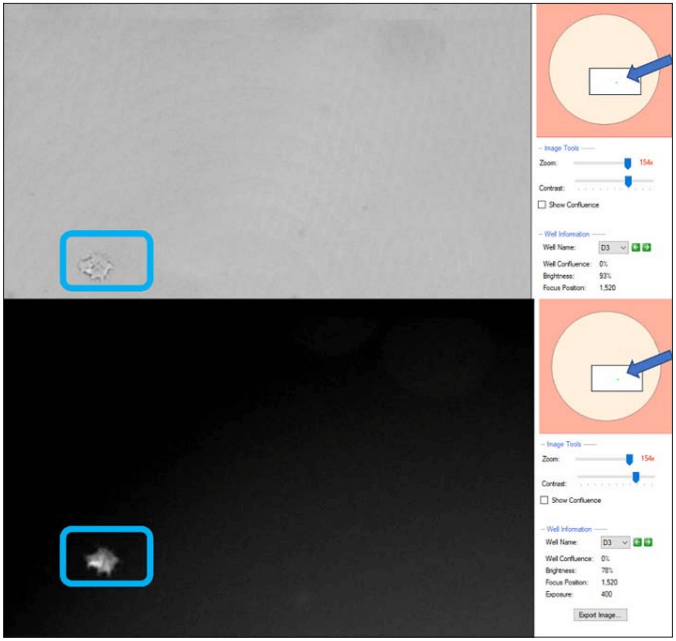
Figure 3. CSI-FL Fusion software white light (top) and RFP (bottom) channel image view of well D3 from plate 1 in monoclonality assurance plate report (Figure 1), on day 6. On the right-hand side of both the images (arrowed), the well layout shows the zoomed image location (boxed) and cells in the well (green spot).
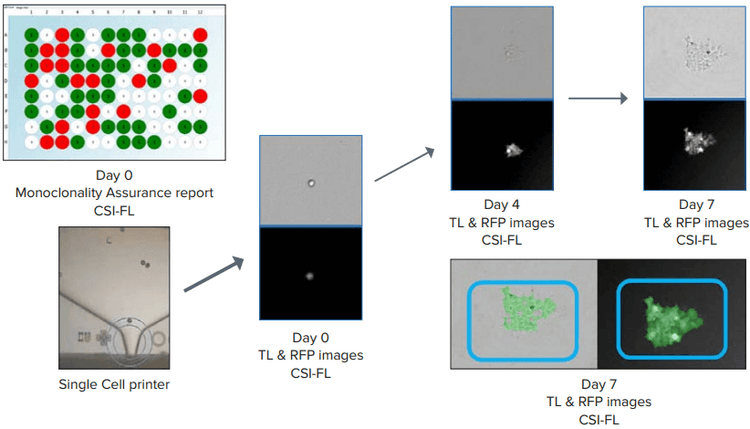
Figure 4. Day 0 monoclonality report for a 96-well plate generated (top left) Single-cell printing (SCP) and cell growth (Day 0 → Day 7) representation. Confluence (blue boxes, bottom right) was generated to count the number of cells using Fusion software. Both white light and RFP images were considered.
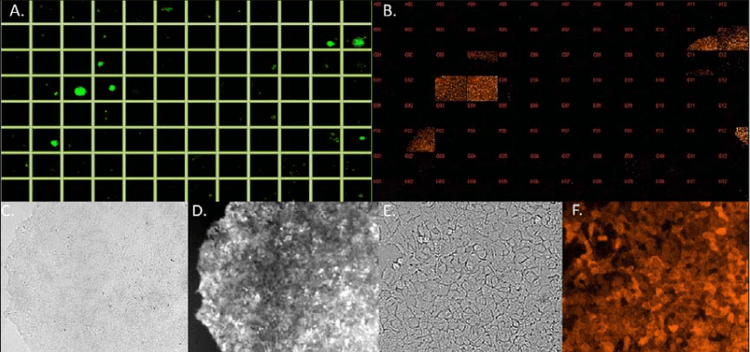
Figure 5. Top – Plate layout images of CRISPR-based p53 KO monoclonal cells with RFP marker from CSI-FL (A.) at 4X and IXM-C (B.) at 10X [Plate 1 on day 11]. Bottom – White light and RFP images of well D3 on day 11 using CSI-FL (4X) (C., D.) and IXM-C (10X) (E., F.), respectively.
Summary
CSI-FL established an effective and rapid process for monoclonal cell-line development with early detection of monoclonality. High-throughput screening of SCP printed cells and assessment for monoclonality on day zero using our fluorescence based monoclonality assurance was achieved. The cells were further expanded using CSI-FL monitoring with automatic confluence analysis. In addition, there was verified RFP expression of clones using fluorescence capabilities all through scale up (96 to 6-well plates). Therefore, the CSI-FL system with its fluorescence screening capabilities helps in the rapid selection of single cells which express the protein of interest (here RFP).