
Application Note
Detect endotoxin with the PyroGene Recombinant Factor C Assay
- Assure sample safety with an endotoxin assay that uses a single enzymatic step and no animal-derived products
- Attain sensitivity beyond minimal requirements with SpectraMax readers
- Save time by automating a read-incubate-read sequence with a workflow in SoftMax Pro Software
Caroline Cardonnel | Sr. Applications Scientist | Molecular Devices
Introduction
Monitoring samples for contaminants is a critical step during the production process in the pharmaceutical and medical device industries. Endotoxin, found in the cell wall of gram-negative bacteria, is a frequent contaminant that can cause fever, inflammation, headache, nausea, and even death. Endotoxin is routinely detected by the Limulus amebocyte lysate (LAL) assay, whose central reagent is obtained from the blood of the horseshoe crab, Limulus polyphemus. In the presence of endotoxins, LAL coagulates via an enzyme-mediated cascade, which can be quantified using turbidimetric or chromogenic assays (Figure 1A). These methods involve multiple enzymatic steps and depend upon the availability of horseshoe crabs.
The PyroGene™Recombinant Factor C (rFC) Assay from Lonza is a quantitative assay that works through a single enzymatic step, uses no animal-derived products, and offers the same sensitivity as the LAL method. In the presence of endotoxin, activated rFC will cleave a fluorogenic substrate, causing an increase in fluorescent signal (Figure 1B) that is proportional to the amount of endotoxin present.
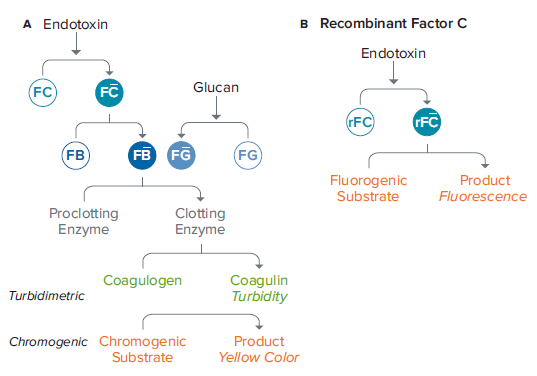
***Figure 1. Enzymatic cascades for the LAL (A) and PyroGene rFC assay (B).*The rFC assay involves a single enzymatic step and eliminates the need for LAL from the horseshoe crab.
Materials
- PyroGene Recombinant Factor C Endpoint Fluorescent Assay (Lonza cat. #50-658U)
- LAL Reagent Grade ™ Multi-well Plates (Lonza cat. #25-340)
- Pyrogen-free Dilution Tubes (Lonza cat. #N207)
- LAL Reagent Water (Lonza cat. #W50-640)
- SpectraMax i3 or i3x Multi-Mode Microplate Reader (Molecular Devices; other SpectraMax microplate readers with fluorescence detection are also suitable)
- SoftMax Pro Software (Molecular Devices)
Methods
The lyophilized E. coli O55:B5 endotoxin supplied with the kit was reconstituted with the volume of LAL Reagent Water indicated on the vial to yield a 20 EU/mL stock solution. The vial was shaken at high speed for 15 minutes using a vortex mixer to reconstitute completely. Standards of the following concentrations were prepared by diluting the stock solution with LAL Reagent Water and shaking vigorously at each dilution: 5 EU/mL, 0.5 EU/mL, 0.05 EU/mL, 0.005 EU/mL, and 0.001 EU/mL. The PyroGene assay has been optimized to be linear from 0.005 EU/mL to 5.0 EU/mL, but the additional standard concentration (0.001 EU/mL) was added in order to test the sensitivity of the SpectraMax i3 reader.
The plate reader temperature was set to 37°C and allowed to reach 37°C before proceeding. 100 μL of blank or endotoxin standard (in triplicate) was added to wells of the microplate. The plate was preincubated in the reader at 37°C for a minimum of 10 minutes.
During preincubation, the working reagent was prepared by mixing fluorogenic substrate, assay buffer, and rFC enzyme solution in a 5:4:1 ratio, respectively. 100 μL of working reagent was added to each well after the 10-minute preincubation step.
The SpectraMax i3 reader temperature was kept at 37°C for the duration of the assay. The plate was read at time zero (after addition of working reagent) using the settings shown in Table 1. Setting the PMT Gain to Automatic in SoftMax Pro Software ensured that accurate results were obtained across the full range of samples without the need to determine a sensitivity setting. Plate height optimization was carried out initially, and the optimized 2-mm read height was used for subsequent measurements.
Ex: 380 nm, bandwidth 15 nm
Em: 440 nm, bandwidth 25 nm
PMT Gain: Automatic
Flashes per read: 6
Read from Top (default)
Read Height: 2 mm
***Table 1. Instrument settings for the SpectraMax i3 reader in SoftMax Pro Software.*Setting the PMT Gain to Automatic negates the need to determine a sensitivity setting for the reader.
After the first (T0) read, the plate was incubated in the reader at 37°C for one hour and then read a second time using the same settings. The read-incubate-read sequence can be automated using the workflow feature in SoftMax Pro Software (Figure 2).
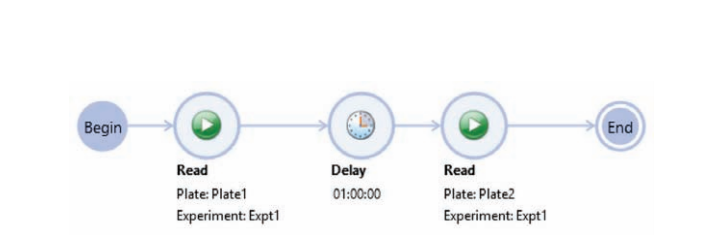
***Figure 2. Workflow setup in SoftMax Pro Software.*The initial read at time zero is followed by a one-hour delay (incubation), and then the plate is read again. The instrument is set to 37°C prior to start of the workflow.
After the second (T60) plate read, net ΔRFU was calculated for each standard using the following formula: (STDT60-STDT0) – (BLT60-BLT0), where…
- STD T60 is the standard RFU at the one-hour timepoint
- STD T0 is the standard RFU at time zero
- BL T60 is the blank RFU at one hour
- BL T0 is the blank RFU at time zero
Results
To demonstrate the suitability of the SpectraMax i3 reader to perform the PyroGene assay, a standard curve was produced by plotting the log of the net ΔRFU vs. the log of the concentration in SoftMax Pro Software (Figure 3).
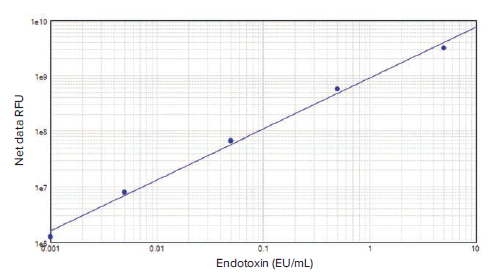
***Figure 3. PyroGene rFC assay standard curve.*The standard curve was generated in SoftMax Pro Software using the log-log curve fit and was composed of five dilutions of control standard endotoxin in duplicate from 0.001 EU/mL to 5 EU/mL (R2=0.996).
The assay was carried out using five endotoxin dilutions ranging from 0.001 EU/mL to 5 EU/mL. The CV for duplicate standards was below 5%. With an R2value of 0.996, the standard curve shows excellent linearity throughout this extended concentration range. The SpectraMax i3 reader was able to detect a lower concentration of endotoxin than the range suggested in the product insert (0.005 EU/mL to 5.0 EU/mL), showing an increased sensitivity for the detection of endotoxin in a fluorescence assay.
Conclusion
The generation of linear standard curves with low variance between replicates, and sensitivity below the suggested lower limit of detection of the assay, demonstrates the excellent performance of the SpectraMax i3 Multi-Mode Microplate Reader with the PyroGene Recombinant Factor C Assay. Similar results can be obtained with the SpectraMax i3x reader and other SpectraMax readers with fluorescence detection.
SoftMax Pro Software was used to analyze data, and the net ΔRFU values calculated were used to plot a standard curve, generate the R2value, and show the sensitivity of this reader in detecting very low endotoxin levels.
Caroline Cardonnel | Sr. Applications Scientist | Molecular Devices
简介
在制药和医疗器械生产过程中,对污染物 样品的监测是一个关键步骤。内毒素存在 于革兰氏阴性细菌的细胞壁中,是一种常 见的污染物,可引起发烧、炎症、头痛、 恶心,甚至死亡。内毒素的常规检测方法 是鲎试剂溶酶法 (LAL),核心成分是鲎的 血液中提取而来。在内毒素存在的情况 下,LAL通过酶促级联凝固,可以通过比 浊法或显色法定量 ( 图 1A )。这些方法涉 及多个酶的步骤,并取决于鲎的可用性。
龙沙公司生产的 PyroGene 重组因子 C 试 剂盒 (rFC) 提供一种定量检测的方法,通 过一步酶促反应,不借助于动物衍生产 品,与 LAL 方法具有相同的灵敏度。在内 毒素存在的情况下,活化的 rFC 会裂解荧 光基质,导致荧光信号的增加 ( 图 1B ), 这与内毒素的含量成正比。

***图 1 酶促级联反应 LAL (A) 和 PyroGene rFC 分析 (B)。*rFC 分析包括一个一步的酶联反应,消除 了鲎对 LAL 的需要。
材料
- PyroGene 重组因子 C 终点法荧光检 测试剂盒 (Lonza cat. #50-658U)
- LAL Reagent Grade™ Multi-well Plates (Lonza cat. #25-340)
- Pyrogen-free Dilution Tubes Lonza cat. #N207)
- LAL Reagent Water (Lonza cat. #W50- 640)
- SpectraMax i3 或 i3x 多功能微孔板读板 机 ( Molecular Devices 公司其它支持荧 光检测的微孔板读板机均可以使用 )
- SoftMax Pro 软件 (Molecular Devices)
方法
用试剂盒提供的冻干的 E. coli O55:B5 内 毒素按照瓶上 LAL 试剂水的体积进行配 置,得到 20 EU/mL 的原液。将配置好的 原液高速震荡混匀 15 分钟,用旋涡搅拌 器使其完全溶解。用 LAL 试剂水稀释原 液,每次稀释时用力摇匀,制得浓度标准: 5 EU/mL、0.5 EU/mL、0.05 EU/mL、 0.005 EU/mL、0.001 EU/mL。经过优化 后 PyroGene 试剂盒检测的线性范围为 0.005 EU/mL 至 5.0 EU/mL,增加了额外 的标准品浓度 (0.001 EU/mL),以测试 SpectraMax i3 读板机的灵敏度。
读板机的温度设置在 37℃,在实验前等待 其内部温度升至 37℃。取 100 µL 空白对 照和内毒素标准品 ( 3 复孔 ) 加入到微孔板 的孔中。该微孔板放置在读板机内 37℃ 孵 育至少 10 min。
在预孵育过程中,分别以 5:4:1 的比例将 荧光基质、检测缓冲液和 rFC 酶溶液混合 制备工作液。在预孵育 10 分钟后取 100 µL 工作液添加到每个孔。
SpectraMax i3 读板机的温度在实验期间 维持在 37℃。使用表一中显示的设置在 0 时刻读板 ( 加入工作液后 )。在 SoftMax Pro 软件中设置 PMT 增益值为自动获取, 可以确保在不需要确定灵敏度设置的情况 下,在整个样本范围内获得准确的结果。 初始进行板高优化,优化后的 2 mm 读高 用于后续检测。
Ex: 380 nm, bandwidth 15 nm
Em: 440 nm, bandwidth 25 nm
PMT Gain: Automatic
Flashes per read: 6
Read from Top (default)
Read Height: 2 mm
***表 1 SpectraMax i3 读板机上 SoftMax Pro 软件的设置。*将 PMT 增益设置为 Automatic,就不 需要为读板机确定灵敏度设置。
在第一次读数 (T0) 后,微孔板在读板机内 37℃ 孵育 1 小时,然后用同样的设置读取 第 2 次。读取-孵育-读取的工作流程可以在 SoftMax Pro 软件设置自动读取 ( 图 2 )。

***图 2 SoftMax Pro 软件的工作流程设置。*第一次读取时间为零,延迟一个小时 ( 孵育 ),然后再次 读取微孔板。在工作流程开始之前,将仪器设置为 37°C。
在第二次读数后 (T60), 每个标准品使用以 下算法计算 ΔRFU:(STDT60-STDT0) ‒ (BLT60-BLT0)
- STD T60 是 1 小时时标准品的 RFU
- STD T0 是 0 时刻时标准品的 RFU
- BL T60 是 1 小时时空白对照的 RFU
- BL T0 是 0 时刻时空白对照的 RFU
结果
SpectraMax i3 读板机满足 PyroGene 试 剂盒的各种要求,使用 ΔRFU 的 log 值和 浓度的 log 值在 SoftMax Pro 软件中绘制 标准曲线 ( 图 3 )

***图 3 SoftMax Pro 软件的工作流程设置。*第一次读取时间为零,延迟一个小时 ( 孵育 ),然后再次 读取微孔板。在工作流程开始之前,将仪器设置为 37°C。
采用五个梯度的内毒素稀释液 ( 浓度从 0.001 EU/mL 到 5 EU/mL ) 进行检测。重 复标准的 CV 低于 5%。R2 值为 0.996,标 准曲线在这一扩展的浓度范围内显示出良 好的线性。SpectraMax i3 读板机能够检 测到比产品说明书中建议的范围 ( 0.005 EU/mL 到 5.0 EU/mL ) 更低的内毒素浓 度,显示其具有更高的荧光检测灵敏度。
总结
标准曲线在低浓度复孔间的变异很小,而 且灵敏度低于建议的检测下限,展现了 SpectraMax i3 多功能读板机在进行 GyroGene 重组因子 C 检测时具有非常优 异性能优势。用 SpectraMax i3x 读板机和 其他荧光检测的 SpectraMax 读板机也可 以得到类似的结果。
SoftMax Pro 软件可用于分析数据,ΔRFU 值用于绘制标准曲线,计算生成 R2 ,并显 示出该读板机在低浓度内毒素检测的灵敏 度。