
Application Note
GeneBLAzer cell-based FRET Assay for G-protein coupled receptors
- Fast read times and improved precision of FRET signals with dual PMTs
- Optimized GeneBLAzer detection cartridge for superb sensitivity
- On-the-fly speed for rapid reads without compromising results
Cathleen Salomo | Applications Scientist | Molecular Devices
Introduction
In this application note, we describe the use of Life Technologies GeneBLAzer assay on the SpectraMax® Paradigm® Multi-Mode Microplate Reader. Best analysis conditions were evaluated using various plate reader settings in black-walled, clear-bottom plates.
GeneBLAzer assay is a fluorescence resonance energy transfer (FRET)-based method to study G-protein-coupled receptors and other drug targets in vivo (living cells) or in vitro (cell lysates). The GeneBLAzer technology is based on ß-lactamase reporter activity in mammalian cells which is detected via a FRET-based substrate (CCF2 or CCF4). The substrate contains two fluorophores: coumarin and fluorescein (Figure 1). When ß-lactamase is not expressed the substrate remains intact. In this state, coumarin is excited and FRET takes place, transferring energy to fluorescein and resulting in the excitation of fluorescein and emission of green light. However, if ß–lactamase is expressed, the substrate is cleaved, the two fluorophores get separated, and energy transfer is disrupted. Now coumarin alone is excited, resulting in emission of blue light. The reporter response is expressed as a blue:green ratio. This normalized, ratiometric measurement results in minimal experimental noise and is less prone to errors, such as variation of cell numbers and transfection efficiency.
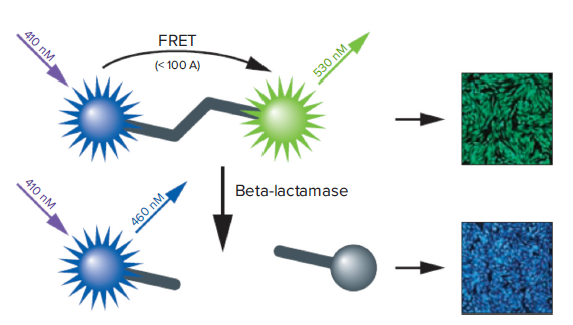
Figure 1. How the GeneBLAzer technology works.
The CCF-2 substrate contains both coumarin and fluorescein. Excitation of coumarin in the intact substrate results in FRET to the fluorescein moiety and emission of green light. With BLA expression, the substrate is cleaved, disrupting energy transfer so that excitation of the coumarin results in a blue fluorescence signal. The resulting blue:green ratio provides a normalized reporter response.
The SpectraMax Paradigm reader offers dual photomultiplier tubes (PMTs) for fast read times and improved precision of FRET signals. The reader’s user upgradeable design allows the configuration of optical detection cartridges according to a user’s needs. This comprises assay-flexible multimode as well as assay-specific cartridges. The Fluorescence Intensity (FI) GeneBLAzer detection cartridge was developed for Invitrogen’s GeneBLAzer assays and includes an ultra-high-power LED for excitation of the FRET donor moiety of the substrate together with an optimized filter set, providing superb sensitivity of the assay. Furthermore, to accommodate HTS applications, the reader supports up to 1536-well microplate formats and can be integrated with the optional StakMax® Microplate Stacker or other robotic platforms. On-the-fly reading enables shorter plate read times while maintaining high data quality.
Materials and Methods
Assay preparation and instrument settings
GeneBLAzer M1 CHO-K1 DA cells (ThermoFisher, K1365) were treated with carbachol (carbamoylcholine chloride, Sigma C4382-10G) to stimulate G-protein activation (Figure 2). The activation stage was measured using the LiveBLAzer-FRET B/G Loading Kit (ThermoFisher, K1095; uses CCF4-AM substrate) on the SpectraMax Paradigm reader. Optimized plate reader settings are shown in Table 1.

Figure 2. Stimulation of G-protein activation in GeneBLAzer CHO-K1 DA cells. Cells were stimulated with increasing concentrations of carbachol, from right to left.
EX: 406-15
EM1: 465-35
EM2: 535-25
Option 1: Stop and go with 140 ms integration time per well
Option 2: On-the-fly performance
Option 3: On-the-fly speed
Table 1. Instrument settings for SpectraMax Paradigm reader with GeneBLAzer Detection Cartridge.
Data analysis
Data and curve fit analysis were performed using SoftMax® Pro Software. Calculations of the blue:green ratio and Z´ factor were determined with the following formulas:
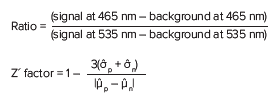
Where p indicates positive control, and n is negative control.
Results
Comparison of plate types
Cells were grown on various plate materials and well densities in order to compare assay performance. Plate characteristics and details are summarized in Table 2. All microplates that were tested achieved comparable EC50 values, with Z' factors above 0.8 (Figure 3). The dose response curve with the highest assay window was observed in the 1536-well SCREENSTAR microplate. A standard tissue culture treatment (CELLSTAR) was sufficient for attachment and growth of the used transfected CHO cells. The SpectraMax Paradigm reader with GeneBLAzer Detection Cartridge was sensitive enough to detect even weak FRET signals, enabling miniaturization of the GeneBLAzer assay to the 1536-well format with no compromise in assay performance. Both 384- and 1536-well plates showed comparable results and can be used with the cell lines and GeneBLAzer reagents tested in this study.
μClear
384-well # 781 091
1536-well # 783 092
SCREENSTAR
384-well # 789836
1536-well # 789866
Table 2. Plate types used.
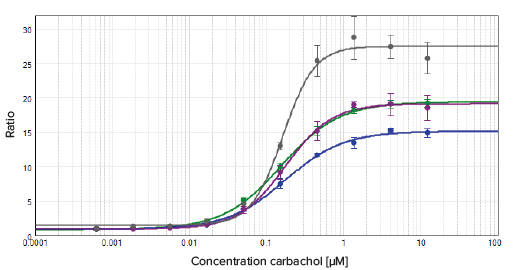
Figure 3. Results comparison using different plate types. Comparing plate types. The data was fitted with a 4-parameter (logarithmic) curve.
Comparison of read speeds
The SpectraMax Paradigm reader offers three options for read speed. All options were tested using the 1536-well μClear plate to determine the fastest possible read time while maintaining acceptable EC50 and Z´ factors.
The following read speeds are available on the reader:
- Stop and go: detection of the signal while no movement of plate occurs and the well centre is positioned under the read head of the plate reader. The signal integration time is set by the user. In this experiment it was set to 140 ms per well.
- On-the-fly: detection of signal in the centre area of the well while plate movement occurs. The signal integration time is fixed.\
- Optimized for speed: designed to obtain the fastest possible read time.
- Optimized for performance: reduces the read speed to increase quality.
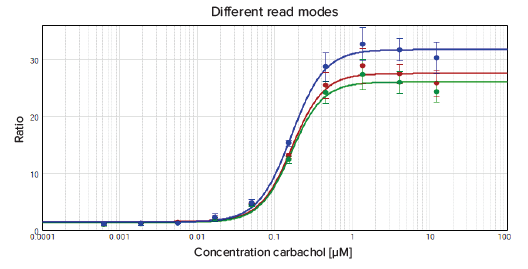
The results are summarized in Figure 4. The EC50 values as well as Z´ factors for the different read modes are comparable. Therefore the fastest possible read speed, On-the-fly optimized for speed, can be used for this application to minimize plate read times and obtain results faster.

Time
Total plate
Read mode
Pattern
Integration time/well
140ms
Figure 4. Comparison of read speeds of the SpectraMax Paradigm reader for 1536-well plates. The data were fitted with a 4-parameter (logarithmic) curve.
Conclusion
This application note demonstrates excellent performance for the GeneBLAzer assay on the SpectraMax Paradigm reader. Assays can be miniaturized to 1536-well format and the fastest read speed can be used to reduce total read time of a 1536 plate to just one minute without compromising the results.
Reference
Zhang, et al., J Biomol Screen, April 1999 Volume 4 No. 2, pp. 67-73.