
Application Note
Improved cell counting method using transmitted light on the SpectraMax MiniMax cytometer
- Improved accuracy of cell counting for difficult-to-image cell types
- Economical alternative to fluorescent cell staining
- Simple workflow from data collection to analysis
Introduction
Caroline Cardonnel, PhD | Field Applications Scientist | Molecular Devices
Andrew Bashford, PhD | Field Applications Scientist | Molecular Devices
Simon Lydford | Field Applications Scientist Manager | Molecular Devices
Hematoxylin, a natural product extracted from the heartwood of the logwood tree (Haematoxylin campechianum), is the most commonly used dye for staining protocols in histology. Hematoxylin reacts like a basic dye with a purplish-blue color and stains acidic or basophilic structures, including the cell nucleus and organelles that contain RNA, such as ribosomes and the rough endoplasmic reticulum1 .
The SpectraMax® i3x Multi-Mode Microplate Reader with the SpectraMax® MiniMax™ 300 Imaging Cytometer is equipped with transmitted light (TL), green fluorescence (Ex/Em: 460/541), and red (625/713) fluorescence channels. The MiniMax cytometer with SoftMax® Pro Software can be used to image and analyze a variety of cells. Here, we demonstrate how hematoxylin can be used to increase the contrast in transmitted light images to improve cell counting for cell lines with relatively flat morphology or indistinguishable intercellular space.
Materials
- Mayer’s Hematoxylin Solution (Sigma cat. #MHS1)
- CellStar 96-well cell culture plate, µClear bottom with black walls (Greiner cat. #655090)
- SpectraMax i3x Multi-Mode Microplate Reader (Molecular Devices cat. #i3x)
- SpectraMax MiniMax 300 Imaging Cytometer (Molecular Devices cat. #5024062)
Methods
Cell culture
MDCK (Madin-Darby canine kidney) cells (parental line, MDCK I, MDCK II) were provide by Dr. James Cooper from the European Collection of Authenticated Cell Cultures (ECACC). They were subcultured in Eagle’s Minimum Essential Medium (EMEM) + 2 mM glutamine + 1% non-essential amino acids (NEAA) + 10% fetal bovine serum (FBS) and maintained at 37°C with 5% CO2 atmosphere in a humidified incubator. Cells were seeded into 96-well plates at 4×104 cells per well and cultured at 37°C for 18 to 24 hours before experimentation.
Hematoxylin staining
The cell media was removed, and Mayer’s Hematoxylin Solution was added directly to the wells to cover the well surface (50 µL per well for 96-well plates) and was left on for 3 to 5 minutes. Then the Mayer’s Hematoxylin Solution was pipetted out of wells, and the cells were gently washed 2 to 3 times with growth media to allow bluing. This step converts the initial soluble red color of the hematoxylin within the nucleus to an insoluble blue color
Data analysis
Images generated in the transmitted light channel were analyzed in SoftMax Pro Software with 'Discrete Object Analysis' (cell count) using either the predefined data analysis setting 'CellsB' or with a new custom data analysis (‘Create New Setting’) to identify and count individual cells. SoftMax Pro Software was used to calculate the number of objects per image. The acquisition and analysis settings are shown in Table 1.
Table 1. Acquisition and analysis settings for the unstained and hematoxylin-stained MDCK cells (all strains). Values are indicated for the plate used here and for these cell types. Other plates and cell types may require different settings.
Results
The MDCK cell lines have an epithelial morphology and are commonly used as a model to study a variety of mechanisms, such as protein trafficking, polarity, and membrane permeability. MDCK cells have also been used more recently to study viral infection of cells, and in vaccine production2. The morphology of MDCK cells depends on the strain3. Parental MDCK cells vary in size and have a heterogenous morphology (Figure 1A). MDCK I cells can be slightly polymorphic with a very fine, almost indistinguishable, cell border (Figure 1B). MDCK II cells are polygonal, with a clearly visible intercellular boundary, which gives to the monolayer a mosaic-like appearance (Figure 1C).
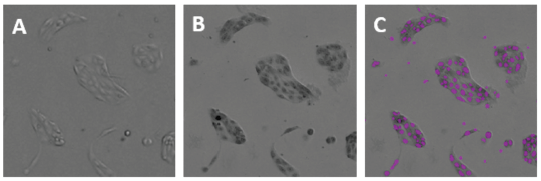
Figure 1. Images of unstained MDCK cells in transmitted light (focus at 10 μm). A: The MDCK parental cell line is polymorphic. B: MDCK I cells have nearly indistinguishable cell membranes. C: MDCK II cells are polygonal with clearly defined cell borders and a mosaic-like appearance.
MDCK II cells could be analyzed with the predefined data analysis setting “CellsB” in SoftMax Pro Software. However, for the other MDCK cell lines, the software could not compensate for the difference in shape and size (MDCK) or for the lack of visibility of the cell membrane (MDCK I) to segment the cells properly.
Upon hematoxylin staining, however, the contrast within the cells increased: cells had a darkened nucleus that could clearly be identified. Hematoxylin-stained MDCK cells were imaged on the MiniMax cytometer in the transmitted light channel, and the resulting images showed an enhanced contrast of the nuclei that enabled them to be identified more easily by the software (Figure 2), while unstained cells were not (Figure 3). This technique was successful for all three strains of MDCK cells: parental (Figure 2), MDCK I (Figure 3), and MDCK II (Figure 4).
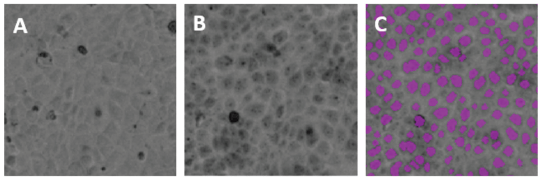
Figure 2. Images of parental MDCK cells from the TL channel of the MiniMax cytometer. A: Unstained parental MDCK cells; B: hematoxylin stained parental MDCK cells; C: same field as B, with cells identified by the software shown as purple masks (overlays).
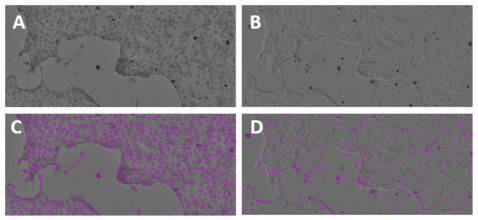
Figure 3. Identification of stained vs. unstained MDCK I cells. Hematoxylin-stained MDCK I cells (A, C) imaged in the TL channel of the MiniMax cytometer could be more accurately identified and counted by the software than unstained cells (B, D).

Figure 4. Unstained and stained MDCK II cells. Hematoxylin-stained MDCK II cells imaged in the TL channel of the MiniMax cytometer were accurately identified and counted by the software.
A new data analysis setting was developed to count all hematoxylin-stained MDCK cells (Table 2). To confirm the robustness of this new method, the cell count obtained was compared with the predefined setting ‘CellsB’ in the transmitted light channel for MDCK II cells in which this analysis works well. The difference in cell count was only about 3% (Table 2), and we confirmed that hematoxylin staining, in combination with the new data analysis, could be used to more accurately count imaged cells and could be a solution to counting flat cells with indistinct intercellular boundaries.
TL
Predefined analysis ‘CellsB’ with unstained cells
TL
New analysis with hematoxylin-stained cells
Table 2. Data analysis for MDCK cells (object count per image is shown).
Please note that hematoxylin staining is incompatible with immunofluorescence, as it abolishes all specific staining through an unknown mechanism4 . When immunofluorescence data are needed, the fluorescence assay should be performed before the hematoxylin staining.
Conclusion
The MiniMax cytometer with integrated data analysis provided by SoftMax Pro Software was used to successfully image and analyze MDCK cell lines in the Transmitted Light channel. Hematoxylin staining increased the image contrast significantly in transmitted light so cells with indistinguishable intercellular space could be counted accurately. This technique provides a solution to count difficult cell lines, such as flat cells or cell clusters. At a fraction of the price of fluorescent stains, hematoxylin is an economical alternative to fluorescence imaging of cells when simple cell counts are desired.
The SpectraMax i3x reader with MiniMax cytometer seamlessly combines the benefits of imaging with the versatility of multi-mode microplate reader assays. SoftMax Pro Software offers a simple workflow from data collection to analysis, and user upgradeable cartridge options make this system a logical addition to any lab.
References
- Titford M. The long history of hematoxylin. Biotech Histochem. 2005 Mar-Apr;80(2):73-8.
- Lugovtsev VY, Melnyk D, Weir JP. Heterogeneity of the MDCK cell line and its applicability for influenza virus research. PLOS One. September 13, 2013. https://pubmed.ncbi.nlm.nih.gov/24058646/. org/10.1371/journal.pone.0075014.
- Dukes JD, Paul Whitley P, Andrew D Chalmers AD. The MDCK variety pack: choosing the right strain. BMC Cell Biology. 2011 12:43.
- Fischer AH, Jacobson KA, Rose J, Zeller R. Hematoxylin and eosin staining of tissue and cell sections. CSH Protoc. 2008 May 1;2008:pdb.prot4986.