
Application Note
Phenotypic characterization of neuroactive compound effects
- Utilize a high-throughput 3D culture platform that more closely resembles the in vivo environment in drug discovery
- Detect functional phenotypic and cytotoxic responses in the same plate
- Analyze calcium oscillation patterns with ScreenWorks Peak Pro 2 software on the FLIPR Penta system
Carole Crittenden | Applications Scientist | Molecular Devices
Oksana Sirenko, PhD | Sr. Research Scientist | Molecular Devices
Cassiano Carromeu, PhD | Director, Research and Development | StemoniX
Introduction
To accelerate the development of effective and safe drugs, there is an increasing need for more complex, biologically relevant, and predictive cell-based assays for drug discovery and toxicology screening. Human iPSC-derived neural 3D co-cultures (StemoniX® microBrain® 3D) have been developed as a high-throughput screening platform that more closely resembles the constitution of native human cortical brain tissue. 3D neural spheroids contain a neural network enriched in synapses, creating a highly functional neuronal circuitry that displays spontaneous, synchronized, readily detectable calcium oscillations.
Here, we describe a method for the complex analysis of calcium oscillations using the FLIPR® Penta High-Throughput Cellular Screening System, which allows detection and multi-parametric characterization of oscillation peaks. ScreenWorks® Peak Pro 2 software analyzes the calcium oscillation rate, peak width and amplitude, characterization of secondary peaks, waveform irregularities, and several other readouts. In addition, we assessed cellular and mitochondrial toxicity by high- content imaging using the ImageXpress® Micro Confocal High-Content Imaging System.
For assay characterization, we used a set of compounds or drugs with known mechanisms of action affecting neurotransmitters such as GABA, NMDA, and dopamine targets. Then we characterized the neurotoxic profile of a set of known neuroactive or seizurogenic drugs, as well as selected environmental chemicals. Our results show that neural 3D cultures, when paired with complex event analysis and cytotoxicity assays, form an accurate, biologically-relevant system for assessing the neurotoxic potential of pharmaceutical drugs and environmental toxins.
Materials
- StemoniX microBrain 3D Assay Ready 384-well plates (StemoniX, cat. #BSARX-AA-0384)
- FLIPR Calcium 6 Assay Kit (Molecular Devices, cat. #R8190)
- FLIPR Penta system with ScreenWorks Peak Pro 2 software (Molecular Devices)
- Cellular viability dye Calcein AM (Invitrogen, cat. #C3100MP)
- Mitochondrial membrane potential dye MitoTracker Orange (Invitrogen, cat. #M7510)
- Hoechst nuclear dye (Invitrogen, cat. #H3570)
- ImageXpress Micro Confocal system with MetaXpress ™ High-Content Image Acquisition and Analysis Software (Molecular Devices)
Methods
Culture and staining of 3D neural cultures
The StemoniX microBrain 3D Assay Ready platform is a high-throughput 3D culture platform that more closely resembles the tissue development and constitution of native human brain tissue. In this platform, human iPSC-derived neural spheroids, approximately 600 μm in diameter, are composed of a physiologically relevant co-culture of functionally active cortical glutamatergic and GABAergic neurons, identified in Figure 1 by MAP2 (green), and astrocytes, identified in Figure 1 by GFAP (red). This balanced cellular mix allows the development of a neural network enriched in synapses, creating a highly functional neuronal circuitry. The neuronal cells in the microBrain 3D spheroids are physiologically active, with spontaneous synchronized, readily detectable calcium oscillations.
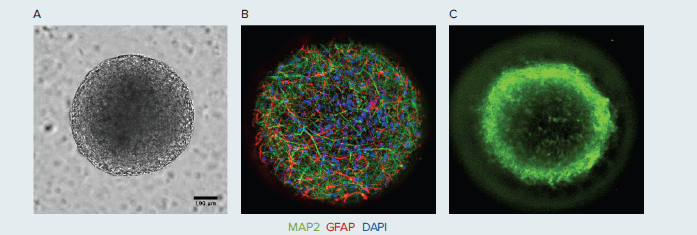
Figure 1. A. Human iPSC-derived neural spheroids composed of a co-culture of B. active cortical neurons (identified by MAP2; green) and astrocytes (identified by GFAP; red), approximately 600 μm diameter, imaged with ImageXpress Micro Confocal system, 20X magnification. C. Stained with Calcium dye.
StemoniX microBrain plates were shipped pre-plated under ambient conditions. Each well contained a single, uniformly sized human iPSC-derived cortical neural spheroid matured 8–12 weeks. Following the manufacturer’s instructions, on the day of arrival, plates were centrifuged for 5 min at 200 x g, inspected by microscope to confirm spheroid settling to the bottom of the plate wells, decontaminated with 70% ethanol, and unsealed. Afterward, the media was changed (1/2 of volume, three times), and plates were placed into a 37°C, 5% CO2 incubator for 5–7 days. Media changes were performed every second day.
Evaluation of calcium oscillation patterns using the FLIPR Penta system
The FLIPR Penta system is powered by a high-speed EMCCD camera and the new ScreenWorks Peak Pro 2 software. The system allows measurement and analysis of complex patterns of calcium oscillations in human iPSC-derived cardiomyocytes and neurons.
We used the new high speed EMCCD camera on the FLIPR Penta system to measure the kinetic patterns and frequencies of calcium oscillations of neural spheroids as monitored by changes in intracellular calcium levels with the FLIPR® Calcium 6 Assay Kit. The system’s ScreenWorks Peak Pro 2 peak analysis software module allowed analysis and characterization of the primary and secondary peaks and complex oscillation patterns.
Intracellular calcium flux in neurons was assessed using the FLIPR Calcium 6 Assay Kit as described in Sirenko, Grimm et al., 2017. Kinetics of intracellular calcium fluxes were determined at 515–575 nm following excitation at 470–495 nm for 10 minutes at a frequency of 2 Hz using the FLIPR Penta system. Camera sensitivity was set to Normal, exposure time per read was 0.05 seconds, read interval was 0.5 seconds, camera gain was set to 6.5, and LED excitation intensity was set to 30%. The instrument temperature was kept at a constant 37°C. Early effects on calcium oscillations were measured at 60 minutes following initial exposure to compounds. For the early time points, cells were preloaded with FLIPR Calcium 6 dye for two hours prior to compound addition. Baseline for calcium oscillations without compounds were measured prior to compound addition. For 24-hour calcium oscillation and imaging experiments, cells were exposed to chemicals at appropriate concentrations for 22 hours prior to the addition of FLIPR Calcium 6 dye. FLIPR Calcium 6 dye (4X concentration) was added for an additional two hours with extra volume of compounds to keep the compound concentration the same.
Assessment of spheroid morphology and viability using the ImageXpress Micro Confocal system
Confocal imaging and 3D image analysis methods were used to characterize compound effects on the morphology and viability of 3D neural spheroids. To evaluate cytotoxicity effects, cells were treated with various compounds for 24h, followed by live cell staining with Hoechst nuclear stain, Calcein AM, and MitoTracker Orange mitochondrial membrane potential dyes. Images were acquired using the ImageXpress Micro Confocal system using the confocal option and 3D imaging.
Results
Calcium oscillations evaluated by the FLIPR Penta system
The neuronal cells in the microBrain 3D spheroids generate spontaneous synchronized calcium oscillations. We used kinetic fluorescence imaging on the FLIPR Penta system to measure the patterns and frequencies of the calcium oscillations of the neural spheroids as monitored by changes in intracellular calcium levels with the FLIPR Calcium 6 Assay Kit. A set of known neuromodulators was tested, including agonists and antagonists of NMDA, GABA and AMPA receptors, kainic acid, and anti-epileptic drugs.
A set of images from calcium oscillations over time recorded on the ImageXpress Micro Confocal system is shown in Figure 2. For quantitative data evaluation, representative descriptors such as peak count (per 10 minutes), average peak amplitude, average CTD 90 peak width (at 90% amplitude from the peak of the oscillation), average peak spacing (time between peaks), average peak rise time (from 90% to 10% amplitude), average peak decay time (from 10% to 90% amplitude), and peak irregularity can be derived using ScreenWorks Peak Pro 2 software. In this experiment, the parameters used were calcium oscillation counts per 10 minutes and peak amplitude. Oscillation patterns vary widely and were also characterized as shown: neurotransmitters in Figure 3, neuroactive compounds in Figure 4, and potential neurotoxic compounds in Figure 5. Representative peak count and peak amplitude concentration response curves are shown in Figure 6. Together with the toxicity data, the parameters tested can be used to create a phenotypic response pattern.

Figure 2. Calcium oscillations over time as seen on the ImageXpress Micro Confocal system.
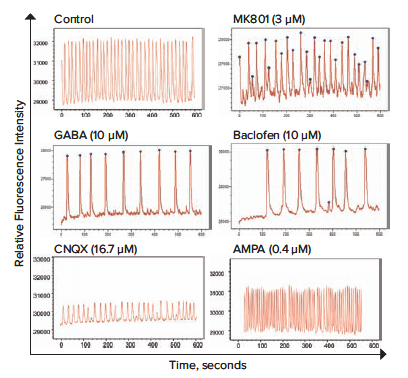
Figure 3. Impact of various neurotransmitters and related compounds on the rates and patterns of intracellular Ca2+ oscillations of neural spheroids using a calcium sensitive dye. Cells were tested using high speed EMCCD fluorescence imaging on the FLIPR Penta system compared with control.
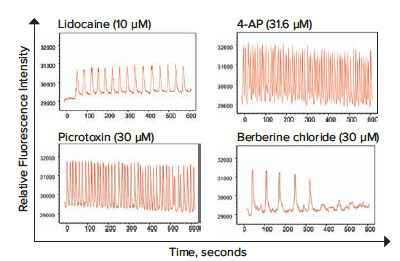
Figure 4. Using the same method as described in Figure 3, three neuroactive compounds were tested to observe phenotypic changes in rate and amplitude of oscillation patterns.
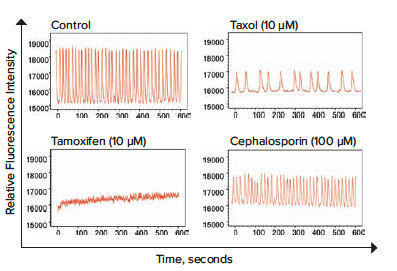
Figure 5. Calcium oscillation patterns from neural spheroids treated with potentially neurotoxic compounds
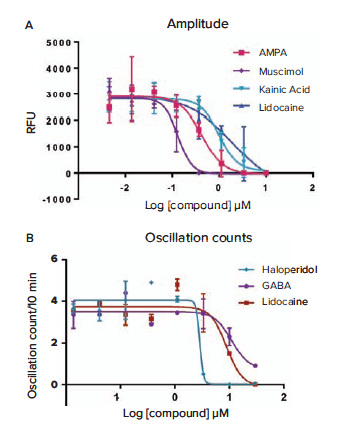
Figure 6a. Concentration response curves based upon average oscillation amplitude derived from some of the compounds tested. IC50 values are: AMPA 0.42 μM, Muscimol 0.14 μM, Kainic Acid 0.98 μM, and Lidocaine 1.9 μM. Figure 6b. Concentration response curves based upon average oscillation counts in 10 minutes from other compounds tested. IC50 values are: Haloperidol \~2.8 μM, GABA 11.1 μM, and Lidocaine 8.9 μM.
Assessment of spheroid viability and morphology by high-content imaging
After treatment with compound, neural spheroids were imaged with the DAPI, FITC, and TRITC 10X Plan Fluor objective and imaged using Z-stack of confocal images (20 images, 10 μm apart). Maximum projection images were analyzed using the Custom Module Editor and Cell Scoring algorithms for detection of cell numbers for all cells, live cells (Calcein AM positive cells), and cells with intact mitochondria (MitoTracker positive cells). Analysis methods provide efficient tools for characterization of cell and spheroid morphology. An example of results for Control and Chlorpromazine can be seen in Figure 7.
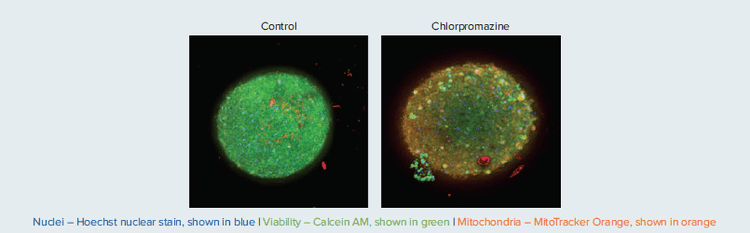
Figure 7. Composite projection images of neural spheroids. Spheroids were treated with 30 μM of indicated compounds for 24h, then stained with a nuclear stain (Hoechst 33342), viability stain (Calcein AM), and mitochondrial membrane potential dye MitoTracker Orange CMTMRos for two hours (2 μM, 1 μM, and 0.5 μM, respectively).
Combining calcium oscillation descriptor data with imaging viability data creates a phenotypic profile of compound effects on neuronal cells in microBrain 3D spheroids. Based upon compound type, EC50 or IC50 values of peak height or peak count where applicable, onset of changes in calcium oscillation by concentration and viability gives details for phenotypic characterization of compound effects (Table 1).
Peak
count
1
Amplitude
(decrease)
1
Secondary
peaks*
Mitochondria
toxicity*
Table 1. IC50 or EC50 values (1) or lowest concentrations (μM) that cause specific changes (*) are indicated for different readouts. No changes indicated with blank cells.
Conclusion
We developed assay methods and have shown the feasibility of using the iPSC-derived StemoniX microBrain 3D Assay Ready neural culture platform for evaluation of compound effects on the FLIPR Penta system. The functional responses from known neuromodulators, neuro-active, and neurotoxic drugs were measured using ScreenWorks Peak Pro 2 analysis software. Combining calcium oscillation peak patterns and early detection of neurotoxicity on the ImageXpress Micro Confocal system created a phenotypic characterization profile useful in high-throughput screening.
For more information
- O. Sirenko, C. Carromeu, et al., Functional and Mechanistic Neurotoxicity Profiling Using Human iPSC-Derived Neural 3D Cultures, Toxicological Sciences, Volume 167, Issue 1, January 2019, Pages 58–76.
- O. Sirenko, FA Grimm, et al., In vitro cardiotoxicity assessment of environmental chemicals using an organotypic human induced pluripotent stem cell-derived model. Toxicology and Applied Pharmacology, Volume 322, 1 May 2017, Pages 60–74.