
Application Note
Live cell Gi- and Gs -coupled GPCR second messenger signaling on the FLIPR Tetra System
- Flexible assay development using GloSensor cAMP cell lines and GloSensor cAMP plasmids
- Complete solution for kinetic screening of the major classes of GPCR subtypes
- Scalable assay throughput for multiple microplate formats with easy integration with automation
Introduction
In this application protocol we demonstrate the use of the modified luminescent firefly luciferase-based Promega GloSensor™ cAMP Assay on the FLIPR Tetra® High Throughput Cellular Screening System to enable detection of cAMP-mediated Gs- and Gi -coupled GPCR activity in kinetic mode. With this assay, these GPCR subtypes can now be evaluated in a live cell assay measuring changes in intracellular cAMP concentration, the relevant second messenger mechanism.
Detection of Gs- and Gi -coupled GPCR second messenger signal activity has been traditionally accomplished using assays such as radioactive binding or endpoint cAMP assays that require cell lysis. Such assays measure activity at a single time point in the cellular response and do not provide kinetic information. Another option utilizes forced-coupling of Gs- and Gi - GPCRs to Gα16 followed by fluorescence detection of calcium flux upon agonist receptor activation. Again, this assay is sub-optimal as it does not signal through the biorelevant cAMP pathway.
We demonstrate endogenous receptor activity in CHO-K1 and HEK 293 cell lines stably expressing the GloSensor plasmid. In addition, stably transfected Gs- and Gi -coupled receptor activity is measured in cell lines transiently transfected with GloSensor plasmid. Combined with the GloSensor cAMP Assay, the FLIPR Tetra System delivers a flexible and complete solution for kinetic screening of the major classes of GPCR subtypes.
As seen in Figure 1, the GloSensor cAMP Assay was created by fusion of a cAMP binding domain to the wild-type N- and C-termini of native firefly luciferase. In the absence of cAMP, the genetically modified luciferase containing the cAMP binding domain is in the inactive state. Upon binding to cAMP, conformational changes in the cAMP binding domain likely determine the increased luminescence in the activated state that can be detected in living cells on the FLIPR Tetra System. Both forms of luciferase are represented in the presence of luciferase substrate. For more information about the GloSensor cAMP assay, refer to the Technical Manual (Cat# TM076, Promega). Flexible Gi - and Gs-coupled GPCR assay design is possible using a combination of plasmids for transient or stable transfections. Four options using either cell lines available from Promega or target cell lines available in the laboratory are listed below:
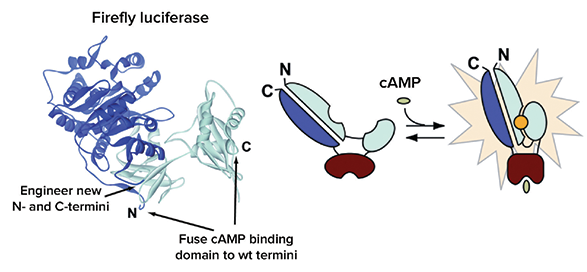
Figure 1. Intracellular biosensor of the GloSensor cAMP Assay. Figure courtesy of Promega Corporation.
Flexible GloSensor cAMP assay design
- Stable receptor and stable GloSensor cAMP assay
- Transient receptor and stable GloSensor cAMP assay
- Stable receptor and transient GloSensor cAMP assay
- Transient receptor and transient GloSensor cAMP assay
About the FLIPR Tetra System:
- Flexible ICCD camera provides luminescent detection for luciferase assay as well as fluorescence detection
- Kinetic cAMP luminescent signal measurements in live cells enabled by the ICCD camera in the FLIPR Tetra System
- Scalable assay throughput: 96-, 384- and 1536-well plate formats, easily integrated with automation
Materials
cAMP cell lines and plasmids
- GloSensor cAMP HEK 293 stable cell line (Promega Corporation, Cat. #E1261)
- GloSensor cAMP 23F CHO K1-stable cell line (Promega R&D)
- Rat Y2R/GloSensor cAMP 23F CHO-K1 double stable cell line (Promega R&D)
- GloSensor cAMP assay plasmid pGlosensor-22FcAMP (Promega, Cat. #E2301)
- Dopamine D4 stable HEK 293T cell line (Multispan Inc., Cat. #C1338)
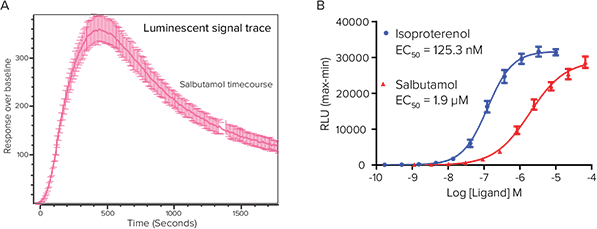
Figure 2. Stable GloSensor cAMP-23F HEK 293 cells. Stable GloSensor cAMP-22F HEK 293 cell line is used to demonstrate the GloSensor cAMP assay on the FLIPR Tetra system. The ICCD camera detects luminescent signal over a period of 30 minutes from modified firefly luciferace. (A) FLIPR ScreenWorks average signal trace of cAMP HEK 293 endogenous Gs- mediated endogenous β2 Adrenoceptor response to salbutamol stimulation. The trace illustrates approximately a 350-fold increase over baseline signal. (B) Full agonism of HEK 293 cell line endogenous β2 Adrenergic receptor in response to isoproterenol and partial receptor agonism by salbutamol.
Cell culture reagents
- HEK 293 cell growth medium: 90% DMEM (Life Technologies, Cat. #11995- 065), 10% FBS (Hyclone, Cat. #SH.30071), 200 µg/mL hygromycin B (Sigma, Cat. #H3274)
- GloSensor cAMP-23F CHO-K1 cell growth medium: 90% F-12 Medium (Life Technologies, Cat. #11765), 10% FBS, and 200 mg/mL hygromycin B)
- Glo Sensor cAMP-23F/Y2R CHO-K1 double stable cell growth medium: 90% F-12 Medium (Life Technologies, Cat. #11765), 10% FBS, and 200 µg/mL hygromycin B), and 500 µg/mL G418 (Life Technologies, Cat. #10131027)
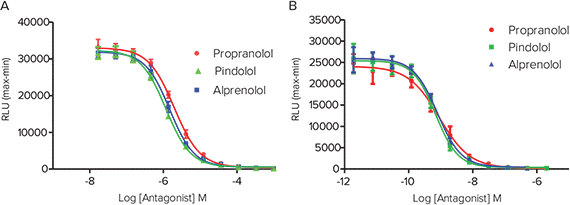
Figure 3. Antagonism of isoproterenol and salbutamol. Antagonism of isoproterenol and salbutamol response by three known inhibitors. (A) Inhibition of EC80 isoproterenol response. (B) Inhibition of EC80 salbutamol response. The robust signal window enables Z factors > 0.7.
Assay reagents
- Plating medium: 90% CO 2 -independent medium (Life Technologies, Cat. #18045), 10% FBS
- HEPES buffer: Re-suspend HEPES in deionized water to 10 mM; adjust pH to 7.5 using KOH
- GloSensor cAMP Reagent stock solution: Re-suspend 250 mg GloSensor cAMP Reagent (Promega, Cat. #E1291) in 8.17 mL of HEPES buffer; store at -70°C in single-use aliquots
- Equilibration medium: Plating medium (above) and 2% or 5% GloSensor cAMP Reagent; optimization is necessary
- Compounds: (-)-isoproterenol (Sigma, Cat. #I6504); salbutamol (Sigma, Cat. #S8260); propranolol (Sigma, Cat. #P0884); pindolol (Sigma, Cat. #P0778); alprenolol (Sigma, Cat. #A8676); dopamine (Sigma, Cat. # H8502). Peptide YY(3-36), (Tocris Cat. #1618); all compound stock solutions made at 10 mM and further diluted in HBSS + 20 mM HEPES
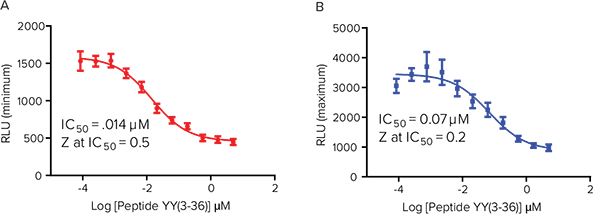
Figure 4. Gi -coupled agonsim results. Gi -coupled receptor agonism results in a reduction in signal correlated with reduction in cAMP inside the cell. Baseline increase in cAMP activity was induced by the addition of forskolin. Using stable P2Y receptor in CHO-K1 cells, we compared results upon addition of forskolin either before or after the agonist. (A) 10 µM forskolin addition followed 15 minutes later by addition of agonist Peptide YY(3-36) on the FLIPR Tetra System. (B) Peptide YY(3-36) was added 15 minutes prior to addition of 10 µM forskolin. In addition to assay flexibility, both methods illustrate a reduction in signal related to inhibited cAMP production.
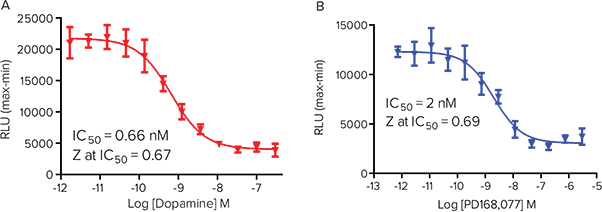
Figure 5. HEK 293 cells over expressing Gi -coupled D4 receptor. HEK 293 cells over expressing Gi - coupled dopamine D4 receptor from Multispan, Inc. were transiently transfectd with GloSensor cAMP- 22F plasmid. Ligand was added on-line to the wells, followed by 5 minute incubation. Continuing the assay, FLIPR Tetra System added 10 µM forskolin to stimulate cAMP production in the cell. (A) Inhibition of forskolin-mediated cAMP production by Dopamine. (B) Inhibition of forskolin-mediated cAMP production by the Dopamine D4 receptor specific compound, PD168,077.
Methods
Stable GloSensor cAMP cell line assay method
Step 1: Cells were plated overnight in 384- well black wall clear bottom plates (Corning, Cat. #3712).
Step 2: Two hours prior to assay, culture media was removed and cells were incubated for 1 hour at 37°C in 5% CO2 and 1 hour at room temperature in 30 µL/well equilibration media containing GloSensor cAMP reagent.
- HEK cell lines were incubated in equilibration media containing 2% GloSensor cAMP reagent
- CHO-K1 cell lines were incubated 5% GloSensor cAMP reagent.
Step 3: 5X compound added by the FLIPR Tetra System during kinetic read.
Step 4: Exposures taken every 10 or 30 seconds for 10–25 minutes.
Step 5: Generally, Gi -coupled receptor assays require longer overall read times due to slower kinetics. Setup parameters for the FLIPR Tetra instrument are seen in Table 1.
Exposure time
*
* Up to 9 sec. exposures may be taken if luminescent signal intensity is lower. Conditions should be optimized.
Table 1. FLIPR Tetra System setup parameters.
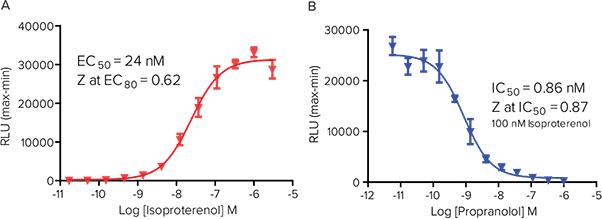
Figure 6. Transient transfection. Transient transfection of GloSensor cAMP-22F and endogenous Gs-coupled cAMP response in HEK 293 cells. (A) Response to isoproterenol and (B) inhibition of the response to 100 nM isoproterenol by propranolol. Results are comparable to those obtained from the experiment performed with the stable GloSensor HEK-22F cell line shown in Figure 2.
Step 6: Data were exported from FLIPR ScreenWorks® Software to Graphpad Prism 5 for analysis.
Transient GloSensor cAMP transfection method in both a stably transfected GPCR receptor cell line and an endogenous receptor cell line
Step 1: Culture cells without selection antibiotics at 37°C and 5% CO2 overnight in flask so that the cells are about 70-80% confluent.
Step 2: Dilute the pGloSensor cAMP plasmid to 20 ng/mL in Opti-Mem- reduced serum media to make 1-µg DNA/mL cells in Step 4. (Life Technologies, Cat. #31985).
Step 3: Add Fugene HD transfection reagent mixture (Roche, Cat. #0409705001) at a 3:1 ratio per one µg DNA and mix by gentle pipetting followed by an incubation of 15 minutes at room temperature into microplate. Reagent is at a 3:1 ratio (µL) : DNA (µG).
Step 4: Add the DNA complex to a tube containing recently lifted cells at 0.48 million per mL (for seeding at 12000 cells/25µL/well for HEK-D4; this is cell linedependent).
Step 5: Depending on the cell line, plate cells at 8000–12000 cells/well in 25 µL/well and incubate at 37°C and 5% CO2 for two days.
Step 6: On the day of the assay, gently remove the culture media and add 30 µL 2–6% f/v GloSensor cAMP Reagent in Equilibration medium.
Step 7: Incubate for 1 hour at 37°C and an additional hour at room temperature.
Step 8: Following the FLIPR Tetra System setup parameters in Table 1, add 6X compound during kinetic read.
Conclusion
Use of the FLIPR Tetra System with GloSensor cAMP assay enables kinetic measurement of Gi - and Gs-coupled receptor signaling not possible using endpoint assays on standard plate readers.
We have shown assay development flexibility using GloSensor cAMP cell lines and GloSensor cAMP plasmids transfected in endogenous as well as stably transfected receptor cell lines.
Acknowledgement
Pete Stecha, Promega Corporation
简介
在这篇应用文献中我们展示了基于 Promega 公司 GloSensor™ cAMP 实验中的修改后的发光萤火虫荧光素酶的应用。在 FLIPR® Tetra高通量筛选系统进行 cAMP 水平的检测以保证在动 力学模式下精确检测 Gi 和 Gs 偶联的 GPCR 活性。通过这样的 实验,基于对细胞内相关第二信使 cAMP 浓度变化的检测可以 对 GPCR 亚型进行评价。Gi 和 Gs 偶联 GPCR 的第二信使信号 活性的检测传统方法一般采用放射性结合或需要细胞裂解的 终点法 cAMP 实验方法。这些类型的实验都是检测一个单独时 间点下的细胞反应和活性,不能提供动力学信息。另一种选 择时采用强制耦合到 G16 的 Gs-和 Gi - GPCRs,检测受体激活 后钙离子流引发的荧光信号检测。然而,这种方法由于不是 直接的反应 cAMP 通路生物相关性信号变化,只能作为第二位 选择。我们展示了稳定表达 Glosensor 质粒的 CHO-K1 和 HEK-293 细胞系中的内源性受体活性。另外,在瞬时转染了 Glosensor质粒的细胞系中检测了稳定转染的Gs-和Gi -偶联受体活 性。结合 Glosensor cAMP 方法,FLIPRTetra 系统建立了一个灵活 的完整解决方案用于 GPCR 主要亚型的动力学筛选。
如图 1 所示,Glosensor cAMP 检测实验主要通过 cAMP 结合域融 合到野生型的萤火虫荧光素酶的 N-和 C-末端来实现。cAMP 缺 乏时,基因修饰过的含有 cAMP 结合域的荧光素酶处于未激活 状态。一旦结合了 cAMP 后,cAMP 结合域构象发生变化处于激 活状态引起化学发光信号增加,从而被 FLIPRTetra系统检测到。 更多关于 Glosensor cAMP 实验的信息可参考技术手册(Cat# TM076, Promega)。
结合质粒瞬时转染或稳定转染的灵活的 Gs-和 Gi -偶联的 GPCR 实验设计是可行的。四种可供选择的来自 Promega 的细胞系或 靶标细胞系见下:

Figure 1. Intracellular biosensor of the GloSensor cAMP Assay. Figure courtesy of Promega Corporation.
灵活的 GloSensor cAMP 实验设计
- Stable receptor and stable GloSensor cAMP assay
- Transient receptor and stable GloSensor cAMP assay
- Stable receptor and transient GloSensor cAMP assay
- Transient receptor and transient GloSensor cAMP assay
FLIPR Tetra 系统:
- 超灵敏 ICCD 相机既可以对化学发光检测,同时 也可以检测荧光信号
- 活细胞中动态的 cAMP 冷光信号检测通过 FLIPRTetra 系统的超灵敏 ICCD 相机来实现
- 实验通量: 96-, 384-和 1536-孔记录板规格,可 很方便与自动化设备整合
材料
cAMP 细胞系和质粒
- GloSensor cAMP HEK 293 stable cell line (Promega Corporation, Cat. #E1261)
- GloSensor cAMP 23F CHO K1-stable cell line (Promega R&D)
- Rat Y2R/GloSensor cAMP 23F CHO-K1 double stable cell line (Promega R&D)
- GloSensor cAMP assay plasmid pGlosensor-22FcAMP (Promega, Cat. #E2301)
- Dopamine D4 stable HEK 293T cell line (Multispan Inc., Cat. #C1338)

Figure 2. Stable GloSensor cAMP-23F HEK 293 cells. Stable GloSensor cAMP-22F HEK 293 cell line is used to demonstrate the GloSensor cAMP assay on the FLIPR Tetra system. The ICCD camera detects luminescent signal over a period of 30 minutes from modified firefly luciferace. (A) FLIPR ScreenWorks average signal trace of cAMP HEK 293 endogenous Gs- mediated endogenous β2 Adrenoceptor response to salbutamol stimulation. The trace illustrates approximately a 350-fold increase over baseline signal. (B) Full agonism of HEK 293 cell line endogenous β2 Adrenergic receptor in response to isoproterenol and partial receptor agonism by salbutamol.
细胞培养试剂
- HEK 293 cell growth medium: 90% DMEM (Life Technologies, Cat. #11995- 065), 10% FBS (Hyclone, Cat. #SH.30071), 200 µg/mL hygromycin B (Sigma, Cat. #H3274)
- GloSensor cAMP-23F CHO-K1 cell growth medium: 90% F-12 Medium (Life Technologies, Cat. #11765), 10% FBS, and 200 mg/mL hygromycin B)
- Glo Sensor cAMP-23F/Y2R CHO-K1 double stable cell growth medium: 90% F-12 Medium (Life Technologies, Cat. #11765), 10% FBS, and 200 µg/mL hygromycin B), and 500 µg/mL G418 (Life Technologies, Cat. #10131027)

Figure 3. Antagonism of isoproterenol and salbutamol. Antagonism of isoproterenol and salbutamol response by three known inhibitors. (A) Inhibition of EC80 isoproterenol response. (B) Inhibition of EC80 salbutamol response. The robust signal window enables Z factors > 0.7.
实验试剂
- Plating medium: 90% CO 2 -independent medium (Life Technologies, Cat. #18045), 10% FBS
- HEPES buffer: Re-suspend HEPES in deionized water to 10 mM; adjust pH to 7.5 using KOH
- GloSensor cAMP Reagent stock solution: Re-suspend 250 mg GloSensor cAMP Reagent (Promega, Cat. #E1291) in 8.17 mL of HEPES buffer; store at -70°C in single-use aliquots
- 平衡溶液:种植液(见上)和 2%或 5% GloSensor cAMP 试剂; 需根据具体实验情况进行优化
- 化合物:(-)异丙肾上腺素(Sigma, Cat. #I6504); 羟甲叔丁肾上腺 素(Sigma, Cat. #S8260); 普萘洛尔(Sigma, Cat. #P0884); 吲哚洛尔 (Sigma, Cat. #P0778); 阿普洛尔(Sigma, Cat. #A8676); 多巴胺 (Sigma, Cat. # H8502). Peptide YY(3-36), (Tocris Cat. #1618); 所有化 合物母液均配置为 10 mM ,添加 HBSS + 20 mM HEPES 做进一步 的稀释

Figure 4. Gi -coupled agonsim results. Gi -coupled receptor agonism results in a reduction in signal correlated with reduction in cAMP inside the cell. Baseline increase in cAMP activity was induced by the addition of forskolin. Using stable P2Y receptor in CHO-K1 cells, we compared results upon addition of forskolin either before or after the agonist. (A) 10 µM forskolin addition followed 15 minutes later by addition of agonist Peptide YY(3-36) on the FLIPR Tetra System. (B) Peptide YY(3-36) was added 15 minutes prior to addition of 10 µM forskolin. In addition to assay flexibility, both methods illustrate a reduction in signal related to inhibited cAMP production.

Figure 5. HEK 293 cells over expressing Gi -coupled D4 receptor. HEK 293 cells over expressing Gi - coupled dopamine D4 receptor from Multispan, Inc. were transiently transfectd with GloSensor cAMP- 22F plasmid. Ligand was added on-line to the wells, followed by 5 minute incubation. Continuing the assay, FLIPR Tetra System added 10 µM forskolin to stimulate cAMP production in the cell. (A) Inhibition of forskolin-mediated cAMP production by Dopamine. (B) Inhibition of forskolin-mediated cAMP production by the Dopamine D4 receptor specific compound, PD168,077.
方法
稳定的 GloSensor cAMP 细胞系实验方法
Step 1. 细胞在 384 孔黑壁底透的细胞板(Corning, Cat. #3712)中过夜培养。
Step 2. 实验前 2 小时,去除培养基并在 37° C 和 5% CO2 条件下培养细胞 1 小时,然后每孔加入含 Glosensor cAMP 试剂的平衡溶液 30 μL 室温下在放置 1 小时。
- HEK 细胞孵育在含 2% Glosensor cAMP 试剂的平衡溶液中
- CHO-K1 细胞孵育在 5% Glosensor cAMP 试剂中
Step 3. 动力学检测过程中通过 FLIPRTetra系统添加 5X 化合物
Step 4. 每 10 秒或 30 秒曝光一次,持续 10-25 分钟
Step 5. 一般来说,由于较低的动力学变化 Gi-偶联的受体实验需要更 长的时间。FLIPR 参数设置见表 1. Step 6. 数据结果从 FLIPR 的 ScreenWorks® 软件输出到 Graphpad Prism 5 进行分析
Exposure time
*
* Up to 9 sec. exposures may be taken if luminescent signal intensity is lower. Conditions should be optimized.
Table 1. FLIPR Tetra System setup parameters.

Figure 6. Transient transfection. Transient transfection of GloSensor cAMP-22F and endogenous Gs-coupled cAMP response in HEK 293 cells. (A) Response to isoproterenol and (B) inhibition of the response to 100 nM isoproterenol by propranolol. Results are comparable to those obtained from the experiment performed with the stable GloSensor HEK-22F cell line shown in Figure 2.
Step 6. 数据结果从 FLIPR 的 ScreenWorks® 软件输出到 Graphpad Prism 5 进行分析
GloSensor cAMP 瞬时转染方法用于稳定表达 GPCR 受体的细胞和内源性受体的细胞
Step 1. 在培养瓶中不加抗生素条件下 37° C 和 5% CO2 过夜培养细胞,密度可达到 70-80%。
Step 2. 在 Opti-Mem-无血清培养基中稀释 pGloSensor cAMP 质粒至 20 ng/mL,使得 step 4 中每毫升细胞体系中含 1-μg DNA/mL (Life Technologies, Cat. #31985)。
染试剂混合物(Roche, Cat. #0409705001),比例是 3:1 每微克 DNA 并轻柔混合后室温下孵育 15 分钟。试剂(μL) 与 DNA (μG)的比例是 3:1。
Step 4. 添加 DNA 复合物至含 0.48 million per mL 细胞的离心管中(种植时 12000 cells/25 μL/well for HEK-D4; 取决于细胞).
Step 5. 根据不同的细胞系, 在每孔 2 5 微 升 体 系 中 种 植 8000–12000 个细胞,并在 37° C 和 5% CO2 条件下培养 2 天。
Step 6. 实验当天,轻柔的去除培养基并添加 30μL 2–6% f/v GloSensor cAMP 试剂至平衡液中。
Step 7. 37° C 孵育 1 小时并在室温下放置 1 小时。
Step 8. 参照表 1 中 FLIPR 系统的参数设置,在动力学检测过程中添加 6X 化合物。
结论
GloSensor cAMP 实验在 FLIPR Tetra 系统上运行,进行 Gi -和 Gs -偶联的 GPCR 受体活细胞的高通量筛选应用。
基于 FLIPR Tetra 系统的 GloSensor cAMP 实验可以进行 Gi -和 Gs -偶联的 GPCR 受体的动力学检测而无需在酶标 仪中进行终点法检测。
通过本篇应用文章,我们展示了使用Glosensor cAMP 细胞系和Glosensor cAMP 质粒转染入内源性受体细胞的实验方 法开发都很方便。